Optional Practical: Density of plasticine
Introduction
In this practical the mass and diameter of different balls of Plasticine will be measured in order to verify that the mass of a sphere is proportional to the cube of its diameter. This is a simple experiment but introduces the concepts of uncertainties, data processing and graphing.
Measuring diameter
Take a piece of Plasticine and roll it into a ball. Try measuring its diameter with a ruler and make a note of it. Turn the ball around and measure it again.
- Is the measurement the same?
- Does the ball have exactly the same diameter in every orientation?
When measuring the ball with a ruler it only measures with an accuracy of about ± 0.2 cm so it isn't sensitive enough to measure small changes in diameter. Using a vernier caliper it is possible to measure the diameter much more accurately, the one in the diagram can measure to ± 0.1 mm.

To read the scale:
- Judge where the 0 on the slider comes ( a little over 4 mm)
- look to see where the sliding scale is in line with the fixed scale ( 0.4 mm)
- The final measurement is these two added together (4.4 mm)
- Note that many vernier calipers are more accurate than this.
You can practice reading the scale with this simulation.
Measure the diameter of your Plasticine ball 10 times with a vernier caliper. Why aren't all of the measurements exactly the same? The uncertainty in your measurement is approximately equal to (maximum diameter - minimum diameter)/2.
- Calculate the uncertainty in your measurements and quote it to 1 significant figure.
- Calculate the mean diameter of the Plasticine ball.
- Write down the diameter and its uncertainty in the following way.

Relationship between mass and diameter
Density is defined as the mass per unit volume 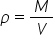
But the volume of a sphere is given by the equation 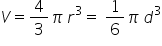
So 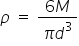
Rearranging this gives 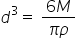
which means that d3 is directly proportional to M. To test this relationship you are going to measure the diameter of many different sized balls of Plasticine. Start with a ball of radius approximately 0.5 cm, measure its mass then measure its diameter 5 times. Repeat this for at least 5 different sized balls entering your data in an Excel spreadsheet like the one below.

- You should decide on your uncertainties yourself, they will depend on the accuracy of your balance and vernier caliper. If using a digital balance then taking the smallest digit as the uncertainty is acceptable.
- The uncertainty quoted for diameter is the uncertainty of the instrument (found written on the caliper), the actual measurement uncertainty will be found from the spread of data.
- Why is the measurement uncertainty greater than the instrument uncertainty?
Processing data
Mean value of d
Now the data has been entered into a spreadsheet it is a simple matter to perform calculations such as finding the average.
- Add header dmean/cm to the next column in your table (To get subscript highlight the text in the formula bar and right click, choose "format cells" and tick subscript).
- Click in the cell at the end of the first row of data.
- Type =ave this will open a list of formula beginning with ave, choose AVERAGE (double click).
- Highlight the row of data that you want to find the mean of.
- Enter
To copy the formula to all the rows
- Select the cell with the formula in it
- Click on the lower right hand corner (you know when you are clicking the right place because the cursor will become a +)
- Drag down to fill all the cells (Excel automatically changes the formula so that it refers to the relevant column).
Calculating dmean3
- Add the header dmean3/cm3 to the next column.
- Type = then click the cell you want to process (in this case it would be G3)) this adds the relevant cell to the formula.
- To calculate d3 type ^3 and enter (the final formula will be =G3^3 but =G3*G3*G3 would also work)
- Copy the formula to all cells in the column as before.
Calculating dmax3 and dmin3
To calculate the uncertainty in d3 you need to know the maximum and minimum possible values. Excel will do this for you with the MAX and MIN functions.
- Add the header dmax3/cm3 to the next column.
- Type =ma and select the formula MAX by double clicking.
- Highlight the values of d.
- Close the bracket and type ^3
- You will probably get an error message (!) informing you that you didn't highlight the cell next to the formula, get rid of this by highlighting the column, clicking the ! and selecting "ignore error"
Now add another column for the minimum value.
Uncertainty in dmean^3
The uncertainty in the mean value of d3 is approximately equal to the difference between the maximum and minimum values.
- Add the header Δdmean3/cm3 to the next column (To get Δ click the "insert" tab and choose Δ from the symbols).
- Write the formula in the first cell to calculate the (dmax3 - dmin3)/2 and copy it to all cells in that column.
- Adjust the number of decimal places in the column so the data is displayed with 1 or 2 significant figure (there is a tool to do this in the toolbar). Use 1 sf if all uncertainties are >0 but 2 sf if some are 0. In this example 2sf are used.
- Adjust the decimal places in the dmean3 column so that it matches the uncertainty.
Graphing Data
You can produce a graph in Excel by highlighting the relevant columns and inserting "scatter chart" however the graphical analysis tools in Excel are limited so in this course we are going to use LoggerPro from Vernier.
Diameter vs mass
- Open LoggerPro.
- Copy and paste the mass column into the first column in the LoggerPro table.
- Copy and paste the dmean column into the second column in LoggerPro.
- Add headers by double clicking the header and filling out the form.

- The graph should plot automatically, if you can't see it click the auto zoom button

- Adjust the axis so that you can see the origin, to do this place your cursor near the axis until you see a wiggly arrow, use this to drag the axis up and down.
- Make the y axis mass and the x axis diameter, to do this click the axis labels and change according.
- To plot the curve click the "curve fit button"
 this will open a form.
this will open a form.

The equation relating m and d is 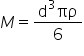 so the curve should be a cubic
so the curve should be a cubic
- Select cubic and then try fit.
- The function will probably not be correct, it will be of the form M = A + Bd + Cd2 + Dd3 . But we know that A, B and C are all equal to 0. You can resolve this by making these constants zero then adjusting D to make the line fit using the + and - buttons
 .
. - The equation will now be of the form M = Dd3 where D = πρ/6. Use your value of D to calculate the density of the plasticine, ρ.
This screen cast maybe easier to follow than the text.
Plotting a straight line (linearising)
In this example, since most of the coefficients (A,B,C) were zero the best fit curve was quite easy to interpret however this is not always the case, for that reason we will often try to linearise the data to plot a straight line that only has an intercept and gradient to explain. Here we have seen that 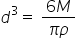 so d3 is directly proportional to M. This means that a graph of d3 against M will give a straight line.
so d3 is directly proportional to M. This means that a graph of d3 against M will give a straight line.
- Copy the M and d3 columns into LoggerPro
- Add headers to the columns (superscripts can be chosen from the drop down menu)
- Make the y axis d3 and the x axis M.
- Adjust the axis to include the origin.
- Add a best fit line by clicking "linear fit"

Adding error bars
To take into account the fact that M and d3 are not exact values we use error bars on the graph to indicate the maximum and minimum values.

- Double click the M header to open up the "manual column options" form.
- Click the options tab and tick the "error bar calculations" box.
- Select "error constant +/- and enter the uncertainty in you measurements.

The values of d3 do not have the same uncertainties so adding error bars to these values is not so simple.
- Create a new column by choosing "new manual column" from the "data" menu.
- Add a header to the new column (Δd3).
- Copy and paste the uncertainty in d3 column from Excel.
- Double click the d3 header and add error bars but this time "use column" and select your Δd3 column.
The best fit line should now hit all of the error bars (either horizontal or vertical).
- According to the equation the gradient of this line should be 6/πρ. Using your gradient calculate the density of Plasticine. Does it agree with the value obtained earlier?
Uncertainty in gradient
Because each point has an uncertainty we can not be sure that the best fit line will give an accurate value of the density. To give an estimate of the uncertainty in the gradient we can plot the steepest and least steep lines that pass through the error bars. This is best shown in a screen cast:
- Plot the steepest and least steep lines then calculate the maximum and minimum values for ρ. The uncertainty in the value obtained from the best fit line is (max-min)/2.
The error bars should reflect the spread of data about the best fit line, due to the approximate way this has been done this is not always the case. LoggerPro can calculate the uncertainty based on this spread, this is often a more realistic value.
- Double click the linear fit label.
- Select "show uncertainty".
- Compare the value obtained with the steepest / least steep values.

