R1.3 Energy from fuels


 R1.3 Energy from fuels (4 hours)
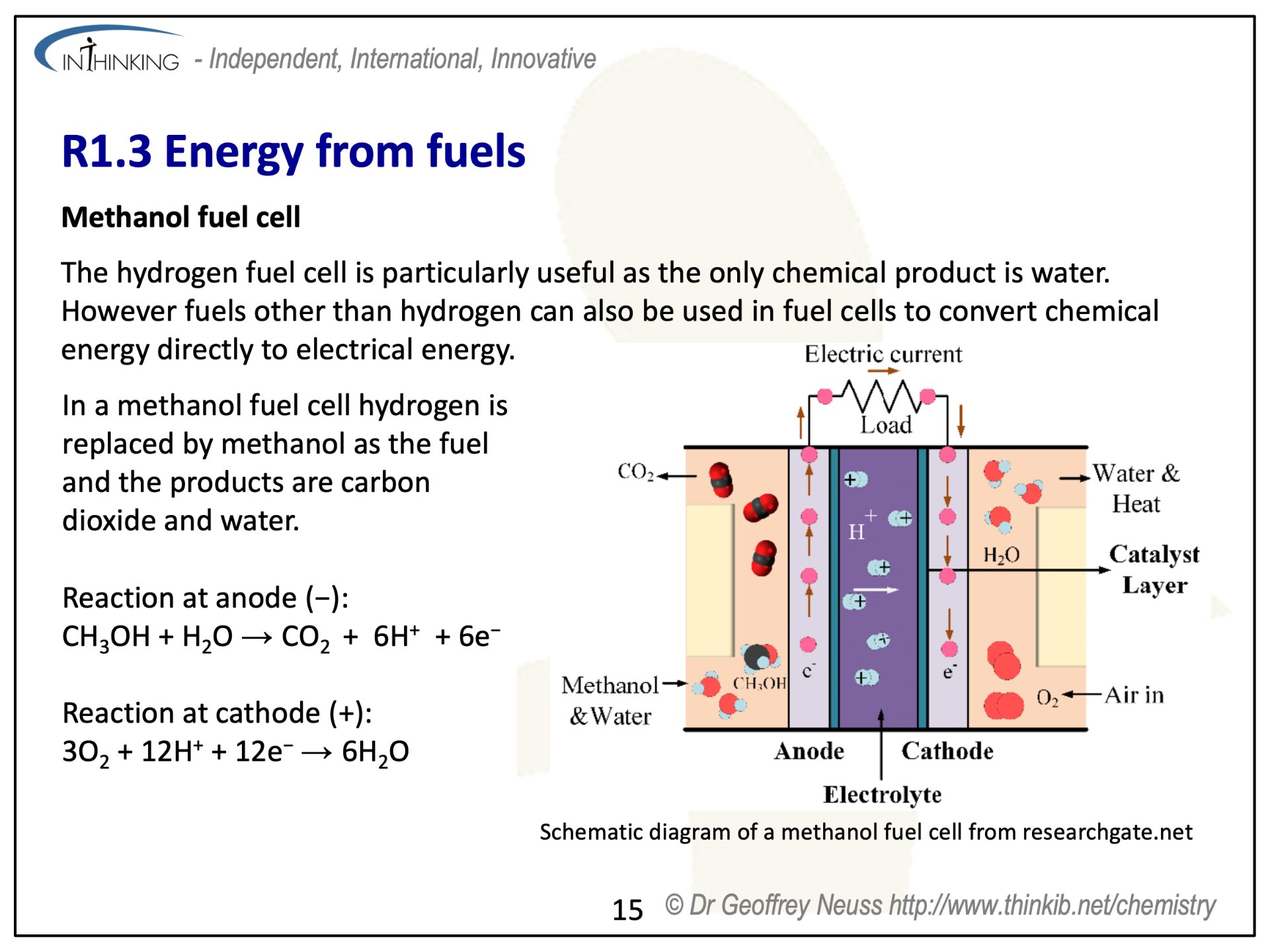
R1.3 Energy from fuels (4 hours)
Guiding question
What are the challenges of using chemical energy to address our energy needs?
Pause for thought
The first crude fuel cells were actually invented as long ago as 1838 by William Grove, a Welshman and independently by Christian Schönbein from Germany.
It was the NASA space programme that really developed them to become a viable technology. The space shuttle contains three fuel cells, which operate as independent power sources. These use oxygen and hydrogen gas and an electrolyte of potassium hydroxide. Each one is capable of supplying 12 kW peak and 7 kW maximum continuous power supply which is ample for the average power consumption of the shuttle. As well as providing electrical power and heat, the product, water, is used by the astronauts for drinking.
.png)
Cut-away of the Toyota Mirai showing the fuel cell stacks beneath the driver and passenger seats.
Only now are fuel cells being seriously used to power cars. The Toyota Mirai, has a range of about 400 miles (650 km) from a full tank. The first cars went on sale in the US in August 2015. The relatively high cost and the current lack of hydrogen fuel stations are the two main initial hurdles that still need to be overcome. Ultimately vehicles powered by fuel cells (FCVs) may well prove to be the best answer to providing transport for the masses that does not rely on fossil fuels and so significantly reduce the emission of polluting greenhouse gases.
Hydrogen fuel cells are used much more widely to power heavy vehicles as the vehicles are more able to accommodate the fuel tanks. The first trains powered by hydrogen fuel cells are also being researched in the UK and made their first journey in September 2020.

Hydrogen powered train
Nature of Science
When a major technology is changed how certain can we be that the new technology will not cause as many (but maybe different) problems as the old one?
Learning outcomesAfter studying this topic students should be able to: Understand:
Apply their knowledge to:
| Clarification notesThe tendency for incomplete combustion and energy released per unit mass should be covered. The reactants and products of photosynthesis should be known. Hydrogen and methanol should be considered as fuels for fuel cells. The use of proton exchange membranes will not be assessed. Linking questionsWhich species are the oxidizing and reducing agents in a combustion reaction? Why is high activation energy often considered to be a useful property of a fuel? What might be observed when a fuel such as methane is burned in a limited supply of oxygen? How does limiting the supply of oxygen in combustion affect the products and increase health risks? Why do larger hydrocarbons have a greater tendency to undergo incomplete combustion? Why is carbon dioxide described as a greenhouse gas? What are some of the environmental, economic, ethical and social implications of burning fossil fuels? What are the main differences between a fuel cell and a primary (voltaic) cell? |
Teaching tipsThis is a rather a ‘bitty’ topic to teach. It starts with the statement that reactive metals and non-metals combust in oxygen but the equations are not necessarily straightforward since burning sodium in air forms about 80% Na2O and 20% Na2O2 and sulfur, which normally forms sulfur dioxide, can be oxidised further in the presence of a suitable catalyst to form sulfur trioxide. Perhaps more to the point none of these is used as a fuel so this sub-topic is only really concerned with the combustion of hydrogen and compounds of carbon as fuels. Make sure your students can write the equations for the complete combustion of hydrocarbons and alcohols. There is a general equation for the combustion of any hydrocarbon, CxHy CxHy + (y/4)O2 → xCO2 + (y/2)H2O but rather than remember this, it is easier to see that there must be xCO2 and y/2H2O formed and then simply balance the O2. Stress upon your students that monohydric alcohols already contain one oxygen atom (this is why they give out less energy than the corresponding alkane) and they should take this into account when balancing the combustion equation for alcohols. Incomplete combustion equations are required but unless the products and their exact amounts are known they are a bit meaningless. Water is always produced; some carbon dioxide may be produced but there must be either carbon or carbon monoxide (or both) included as the incomplete combustion products. Distinguish between non-renewable fossil fuels (coal, crude petroleum oil and natural gas) and renewable biofuels. It can be a little hard to know exactly what to include. Fossil fuels are often converted into other fuels e.g. synthesis gas etc. and crude petroleum can undergo cracking and reforming to form more useful products. The exact composition of gasoline varies according to its octane number (ability to reduce ‘knocking’) etc. Biofuels may be used directly (e.g. wood) or they may be fermented to form alcohols or broken down anaerobically to produce methane. None of these refinements are listed on the syllabus so students do not appear to be required to know any specific details but they should perhaps be aware of their existence. With most countries now signed up to be carbon neutral by 2050 or earlier, battery powered electric cars are gaining in use but perhaps the longer term solution is the use of fuels cells. The coverage required here though is rather factual with the focus on the half-equations rather than how fuel cells are manufactured. Stress too that the fuel and oxygen needs to be continuously supplied. The hydrogen fuel cell is non-polluting as the only product is water. | Study guide:
Pages 80 − 83 Questions
For ten 'quiz' multiple choice questions with the answers explained see MC Test: R1.3 Energy from fuels. For short-answer questions see R1.3 Energy from fuels questions together with the worked model answers. Vocabulary listspecific energy Putting the topic into contextSee Topic R1 What drives chemical reactions? which covers all of Topic R1. Practical work
|
Teaching slides
Teachers may wish to share these slides with students for learning or for reviewing key concepts.
Other resources
1. Ten future energy sources that can replace fossils fuels.
2. A Guardian video giving three good reasons as to why we need to keep fossil fuels in the ground.
Why we need to keep fossil fuels in the ground
3. A useful site from Energy4me comparing the pros and cons of different energy sources.
4. The Green Ninja advises on how to reduce your carbon footprint.
Green Ninja: Footprint renovation
5. An animated overview of fuel cells by the Naked Science Scrapbook which provides a good introduction to the different types of fuel cells and why they are useful (but does not include the half-equations).
6. A good classroom exercise from NASA on fuel cells.

You can download the student worksheet for this exercise and also the accompanying teacher's sheet.