Teaching students to think critically
The need to analyse data critically
A simple example of critical thinking
A few years ago, Tesco’s, a large UK supermarket chain, put out a statement about its Christmas trading called ‘Tesco’s Christmas in numbers’.

Part of the statement said:
“Tesco will also sell around 3.5 million packs of carrots – that’s 27 million carrots! If these were to be placed in a line it would stretch 11 times around the world”.
That’s impressive. However it is even more impressive if you analyse it critically.
The circumference of the Earth is 40,075 km so eleven times the circumference of the Earth is 11 x 40,075 = 440,825 km. If the 27 million carrots are laid end to end then this means that the average length of a Tesco’s carrot is 440,825 ÷ (2.7 x 107) = 0.0163 km = 16.3 metres (or about 53 feet). Now that is impressive!!!
This simple example shows how students should not just accept what they read, however authoritative the article purports to be, but analyse all the data and other information they receive critically for themselves. In a world where elections can be fought and won on ‘false’ or ‘fake’ news and the increasing use of artificial intelligence it is our responsibility as teachers to encourage students to think critically about all the facts they are presented with. Our specific remit is to help them apply critical thinking to Chemistry. However, the spin-off is it that it will also help them question critically information they receive in many other areas (both academic and life in general) where they need to base decisions on correct facts, correct interpretation of data and non-fallacious arguments.
Defining 'critical thinking'
There have been many attempts to define critical thinking. Perhaps one of the simplest from dictionary.com is: "the process of actively and skilfully conceptualising, applying, analysing, synthesising, and evaluating information to reach an answer or conclusion" In many ways “evaluating information” is the bedrock upon which critical thinking is based. If students cannot evaluate information, they cannot think critically. This is important because one way in which students can impress external examiners for both their Internal Assessment and Extended Essay and demonstrate that they have analysed the sources they have used is to point out errors and/or inconsistencies they have found in them. So how can we train them to think critically? Perhaps one of the best ways is to provide them with concrete examples and ask them, either working on their own, in pairs or as a group, to question critically the information they are presented with. I’ve tried to illustrate this with examples throughout the whole of this site, mainly through the ‘Pause for thought’ sections. It might be helpful to concentrate just a few of these examples onto a single page. I appreciate that unfortunately many sources of information do contain “typos’ (I know I am guilty of these myself at times) but the examples below are those where the basic chemistry is simply wrong.
Website examples
1. The following is taken from an article produced by The Department of Food Science and Technology at Virginia Tech. It is often cited by students who are doing an extended essay involving the chemistry of wine making.

Can you identify two major errors?
In Step 1 an oxygen atom has disappeared from one of the rings and in Step 2 the equation is unbalanced.
2. The following question can be found on a question sheet providing problems on equilibrium which students are likely to encounter during their first year at university. Problem 1 and the solution given are very much on the AHL syllabus under Topic R3.2.6. Ask your students to look at the problem critically.

There are several minor errors (we are all guilty of these at times!). For example, the article is by Taylor and Crist (not Krist) and the value in the answer given for the concentrations of the hydrogen and iodine should be (1.00 − 0.777) = 0.223 not 0.233. The temperature should also be given as 730.8 K not 730.8 oK.
However the major error is that the answer is completely wrong! When hydrogen iodide dissociates the amount in moles on both sides of the equation does not change. If the initial amount of hydrogen iodide is n moles then the total amount of moles at equilibrium = n moles, not (n + 0.223n) as given here. From the stoichiometry, when n moles of HI dissociates it will give n/2 moles of hydrogen and n/2 moles of iodine. The first sentence in the solution should read "No explicit molar concentrations are given, but we do know that for every n moles of HI, 0.1115n moles of each product is formed and (1 − 0.223)n = 0.777n moles of HI remains."
The answer should be:
Kc = (0.1115)2 ÷ (0.777)2 = 0.021
3. Consider the article on biodiesel production from waste cooking oil that appeared in the Journal of Environmental Protection in 2011 and was presumably peer reviewed. It included the following equation to illustrate transesterification (which is a way of converting used cooking oil into diesel fuel):
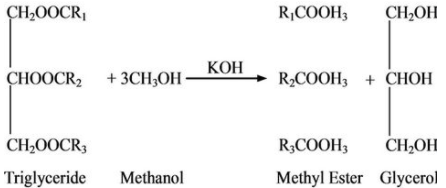
You could ask your students to comment on it.
All three methyl esters in the products are missing a carbon atom, i.e. the first one should have the formula R1COOCH3
Text book example
The following diagram is taken from taken from G. Hill and J. Holman, Chemistry in Context, 5th edn, Nelson, 2000).
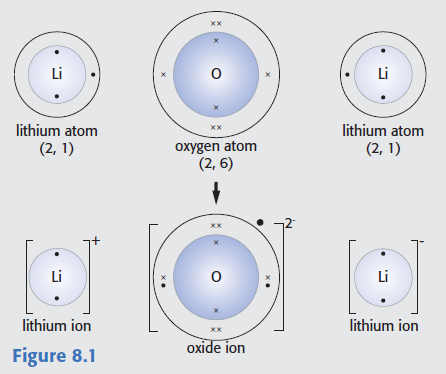
Identify three major errors.
Oxygen atoms have a smaller atomic radius than lithium atoms, oxygen ions are much bigger than oxygen atoms and the lithium ion on the right should have a positive (rather than a negative) charge.
For more on this example see my Data response question which covers many of the errors often made when ionic bonding is covered.
Video example
Ask you students to watch the video below produced by Ohio State University on estimating the enthalpy of combustion of propane from bond enthalpies.
Hopefully your students will realise that the value of -3361 kJ mol-1 obtained in the video by using average bond enthalpies is very different to the literature value of -2219 kJ mol-1 given in Sectiom 14 of the IB data booklet. Ask them to analyse critically why the result obtained in the video is wrong.
The main error is that it ignores the change of state from gaseous water to liquid water.
For more on this example see 'Other resources' on my page on R1.2 Energy cycles in reactions.
Newspaper example
The following extract is from an article in the South Wales Post and also reported on the BBC.

Should you be worried if your school has DNP in your chemical storeroom?
No. DNP is the shortened version of 2,4-dinitrophenol which is not explosive. It is not the acronym for 2,4-dinitrophenylhydrazine. The shortened version of 2,4-dinitrophenylhydrazine is 2,4-DNP or 2,4-DNPH.
IB Chemistry Guide example
Even the IB chemistry guides and examination papers are worth looking at critically.

For example, the guide for first examinations in 2016 under Alcohols in 10.2: Functional Group Chemistry stated:
"Alcohols undergo esterification (or condensation) reactions with acids and some undergo oxidation reactions."
Analyse this statement critically.
There are two potentially misleading or incomplete concepts in this statement.
Alcohols do not undergo condensation reactions with all acids. The only acids they react to form esters with are carboxylic acids. They do not undergo condensation reactions to form esters with strong mineral acids such as hydrochloric acid, nitric acid or sulfuric acid. They also do not form esters with other organic acids such as phenol (carbonic acid), picric acid (2,4,6-trinitrophenol) or sulfonic acids (e.g. 4-methylbenzenesulfonic acid, CH3C6H4SO3H).
The second statement implies that only some alcohols undergo oxidation reactions. In fact they all do - just set fire to them. All alcohols combust in a plentiful supply of oxygen to form carbon dioxide and water. What is missing here is "and retain their carbon chain" as only primary and secondary alcohols can be oxidised without breaking up the carbon chain.
IB examination papers examples
For examples from examination papers see my two blogs Thinking through multiple choice questions and Some thoughts on the May 2022 IB Chemistry examinations.
You can find many more examples where I have challenged "accepted wisdom" by analysing information critically on individual pages throughout the website. Often this links directly to the Nature of Science - see for example NOS & Critical Thinking.
