Concept-based assessment
Sunday 13 November 2022
 There is an interesting question on the 2022 November examination paper which is worth exploring particularly as the IB moves toward concept-based learning and assessment which will be embodied in the new Guide due to be published early next year.
There is an interesting question on the 2022 November examination paper which is worth exploring particularly as the IB moves toward concept-based learning and assessment which will be embodied in the new Guide due to be published early next year.
The question states that chloroquine can be synthesised by reacting 4,7-dichloroquinoline with another compound labelled B and asks students to identify B.

From one point of view this seems a ridiculously easy question and yet from another point of view it is extremely hard and requires a knowledge and understanding of chemistry that is clearly way beyond the IB syllabus.
Broken down simply the question shows the replacement of the halogen in R−Cl by −NHR' so an obvious answer which requires almost no understanding or application of the underlying chemistry is that B is R'NH2 and HCl will be released. i.e. the structure of B, which I presume the IB is expecting as an answer, is:
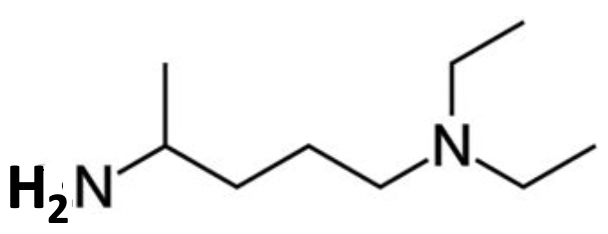
As teachers though we should be encouraging our students to solve problems like this by understanding and applying the underlying chemical concepts they have learned during their IB chemistry diploma course.
Students are very unlikely to have come across the structures and chemistry of chloroquine (an anti-malarial drug) and 4,7-dichlorchloroquinoline before. This, in itself is not a problem as IB students should be able to apply their knowledge to unfamiliar situations. In an exam they would not be able to question the initial statement but in fact the reaction is just the final step in the synthesis of chloroquine from simpler starting materials. From their knowledge students should be able to deduce that this reaction can be described either as a condensation reaction (which is how it is described in one of the early research papers on this synthesis https://pubs.acs.org/doi/pdf/
The only mention of condensation reactions in the core/AHL is in Topic 10.2 which covers the condensation of alcohols with carboxylic acids in the presence of a catalyst such as concentrated sulfuric acid (i.e. esterification). There is no mention of condensation in the AHL (Topic 20). The only other mention of condensation reactions is in the options. Option A covers condensation polymers at AHL in sub-topic A.9 and the formation of polypeptides is covered in Option B. However, this is a Paper 2 question so knowledge of the option content is not being assessed. Even if it was, the only knowledge an IB student would have of condensation reactions involving amines is that it occurs when they react with an acid chloride or a carboxylic acid group (e.g. nylon and peptide bond formation). They would not be able to deduce that amines could undergo a condensation reaction with a chloroarene, let alone one that also contains a nitrogen atom in the aromatic ring.
The IB syllabus only refers to the nucleophilic substitution reactions of halogenoalkanes and the only nucleophile actually mentioned is the hydroxide ion. Nowhere are nucleophilic reactions involving halogenoarenes covered on the syllabus and amines are not listed as nucleophiles. The only reactions of arenes covered on the syllabus core/AHL are the addition reaction of benzene with hydrogen (as evidence for resonance/delocalisation) and the electrophilic substitution nitration of benzene using concentrated nitric acid and concentrated sulfuric acid where the nitronium ion, NO2+ acts at the electrophile. As it happens in my practical 'Hydrolysis of halogenoalkanes' I do get students to try to react bromobenzene as well as various isomers of bromobutane with a nucleophile even though it is not on the syllabus. Students can deduce that the non-bonding pair of electrons on the bromine atom delocalise into the aromatic ring making the C–Br bond stronger (i.e. a poorer leaving group) which, together with the repulsion by the pi electrons, means that halogenoarenes are not easily susceptible to nucleophilic substitution.
What this shows is that knowledge and understanding of IB chemistry will not help students to answer this question and in fact it will hinder them as they would not expect a chlorine atom on an aromatic ring to be substituted by an amine. From Topic 19 students are required to explain why the hydroxide ion is a better nucleophile than water. A really good student who does reason that this question is about nucleophilic substitution would predict that B is actually a substituted amide ion as this negatively charged ion will be a better nucleophile than the neutral amine molecule so I hope the IB will also accept Na+RNH− as a correct (actually better) answer. The other product in the reaction, which is not given in the question, would then be NaCl rather than HCl.

The sodium salt is a better nucleophile (so is a better answer?) than the neutral amine.
As an aside the next question on the exam paper, (e)(ii), would appear to follow on. It states "The reaction can be done (sic) using a copper catalyst. State the ground state electron configuration for copper." The electron configuration of copper is clearly on the syllabus (Topic 2.2) but how does this question and the answer required relate to how copper acts as a catalyst in the chloroquine synthesis. This is not concept-based learning and assessment.
Although beyond the IB syllabus, if you do want a good follow on question and you have got some excellent students, ask them to discuss why the substitution of the chlorine atom occurs at the 4-carbon atom and not the 7-carbon atom.

